Preparation of blood smear (Blood Film)
Blood smear (Blood film )
• The blood film or peripheral blood smear is a thin layer of blood that is spread on a slide and then stained so that different blood cells can be examined under the microscope.
The objective of blood smear
• The blood film is usually checked to investigate hematological problems (blood diseases), and occasionally the blood is checked for parasites such as malaria and filariasis
• Examination of thin blood films is principal in the investigation and treatment of anemia, infections, and other conditions that produce changes in the appearance of blood cells and differential white cell count.
• A blood film report can give rapidly and at low cost, useful information about a patient’s condition.
The Ideal Stained Smear can provide:
— Estimates of cell count
— Proportions of the different types of WBC
— Morphology
Types of the peripheral blood film (PBF):
• 1. Thin blood film
• 2. Thick blood film
Three basic steps to make blood film:
• 1. Preparation of blood smear.
• 2. Fixation of blood smear.
• 3. Staining of blood smear.
Preparation of blood smear
1) Thin Blood Film
Thin PBF can be prepared from anticoagulated blood obtained by venipuncture or from free-flowing finger-prick blood by any of the following three techniques:
1. Slide method
2. Cover glass method
3. Spin method
Slide Method
Procedure
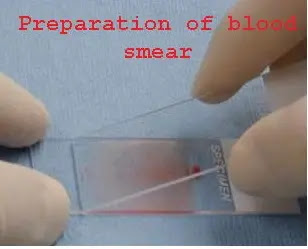
• Place a drop of blood in the center of a clean glass slide 1 to 2 cm from one end.
• Place another slide (spreader) with a smooth edge at an angle of 30-45⁰ close to the drop of blood.
• Move the spreader backward so that it contacts drop of blood.
• Then move the spreader forward rapidly over the slide.
• A thin peripheral blood film is therefore prepared
• Dry it and stain it.
Cover Glass Method
Procedure
• Take a clean cover glass.
• Touch it on to the drop of blood.
• Place it on another similar cover glass in a crosswise direction with the side containing a drop of blood facing down.
• Pull the cover glass quickly.
• Dry it and stain it.
• To preserve it use the mountant to mount the slide, film side down on a clean glass slide.
Spin Method
This is an automated method.
Procedure
• Place a drop of blood in the center of a glass slide.
• Rotate at a high speed in a special centrifuge, cytospin.
• Blood spreads uniformly.
• Dry it and stain it.
2. Thick Blood Film
This preparation is used for detecting blood parasites such as malaria and microfilaria.
Procedure
• Place a large drop of blood in the center of a clean glass slide.
• Spread it in a round area of 1.5 cm with the help of a stick or end of another glass slide.
• Dry it and Satin it.
Steps for blood film
The shape of the blood film
Qualities of a Good Blood Film
1. It should not cover the entire surface of the slide.
2. It should have a smooth and even appearance.
3. It should be free from waves and holes.
4. It should not have an irregular tail.
The thickness of the spread is determined by:
1. The angle of the spreader slide. (greater angle produce thick and short smear).
2. Size of the blood drop.
3. Speed of spreading.
Notes:
1. If the hematocrit increased, the angel of the spreader slide should be decreased.
2. If the hematocrit decreased, the angel of the spreader slide should be increased.
Parts of a Thin Blood Film
A peripheral blood film consists of 3 parts:
1. Head: The part of the blood film closes to the drop of blood.
2. Body: The main portion of the blood film.
3. Tail: The narrow end of the blood film.
The common cause of a poor blood smear:
1. A drop of blood too large or too small
2. Spreader slide pushed across the slide in a jerky manner.
3. Fail to maintain the whole edge of the spreader slide against the slide while making the smear
4. Fail to keep the spreader slide at a 30⁰ angel with the slide
5. Fail to push the spreader slide completely across the slide
6. Asymmetrical spread with ridges and long tail: edges of spreader dirty or chipped; dusty slide
7. Holes in the film – slide contaminated with fat or grease and air bubbles
8. Cellular degenerative changes: delay in fixing insufficient fixing time or methanol contaminated with water.
A. Blood film with a jagged tail made from a spreader with a chipped end.
B. The film is too thick
C. The film is too long, too wide, uneven thickness, and made on a greasy slide.
D. A well-made blood film.
Biologic causes of a poor smear:
1. Cold agglutinin - RBCs will clump together.
Warming the blood at 37° C for 5 minutes, and then remake the smear.
2. Lipemia - holes be seen in the smear.
There isn't anything you can do to fix this.
3. Rouleaux - RBC’s will form into stacks resembling coins. There isn't anything you can do to fix this.
Fixation of blood smear
• To keep the morphology of the cells, films must be fixed as soon as possible after they have dried.
• It is necessary to prevent contact with water before fixation is complete.
• Methyl alcohol (methanol) is the main option, although ethyl alcohol ("absolute alcohol") can be used.
• Methylated spirit (95% ethanol) never be used as it contains water.
• To fix the films, put them in a covered staining jar or tray containing the alcohol for 2-3 minutes. In humid climates it might be essential to replace the methanol 2- 3 times per day; the old portions can be used for storing clean slides.
Staining of blood smear
Various stains for peripheral blood film:
- Romanowsky stain is commonly used for blood film staining. All Romanowsky combinations have two essential ingredients i.e. methylene blue and eosin or azure.
• Methylene blue is the basic dye and has an affinity for the acidic component of the cell (i.e. nucleus) and eosin/azure is the acidic dye and has an affinity for the basic component of the cell (i.e. cytoplasm).
• Most Romanowsky stains are prepared in methanol, so they combine fixation and staining.
- Various stains included under Romanowsky stain are as under:
1. Leishman stain
2. Giemsa stain
3. Wright stain
4. Field stain
5. Jenner stain
6. JSB stain
Leishman Stain:
Preparation
• In a conical flask, 0.2 g of powdered Leishman dye was dissolved in 100 ml of acetone-free methanol
• Shake occasionally and heat it to 50°C for half an hour.
• Cool it and filter it.
Procedure for staining
• Pour Leishman's stain on the slide drop by drop (count the droplets) and wait 2 minutes. This can fix PBF in methanol.
• Add twice the amount of buffer water to the slide (ie, double the number of drops).
• Mix by rocking for 8 minutes.
• Rinse with water for 1-2 minutes.
• Dry in air and examine under the oil immersion lens of the microscope.
Giemsa Stain
Preparation
• A stock solution of Giemsa dye was prepared by mixing 0.15 g of Giemsa powder with 12.5 ml of glycerin and 12.5 ml of methanol.
• Prior to using, dissolve one volume of the stock solution in nine volumes of buffer water (dilution 1:9).
Procedure
• Fill staining dish with staining solution
• Put thin film and thick films into the staining dish.
• Stain blood slides for 45 minutes
• Wash in water.
• Dry it and examine under the oil immersion lens of the microscope.
Caution:
The thick film needs to be rinsed carefully (because it is not fixed before staining)
Mistake:
Thick films are not rinsed properly! Blood is lost.
Wright's stain:
• Wright's stain is used to distinguish the nuclear and/or cytoplasmic morphology of platelets, red blood cells, white blood cells, and parasites.
Procedure
• Thin blood films (only) – Dip Method
1. Dip air-dried blood film in the undiluted stain for 15 to 30 seconds (double the staining time for bone marrow smears).
2. Remove the color of the stained smears by immersion in distilled or deionized water and air dry
3. Let air dry in a vertical position.
• Thin blood films (only) – Rack Method
1. Lay air-dried slides on a staining rack and flood with stain; stain for 10 to 15 seconds (double the staining time for bone marrow smears).
2. Add a similar volume of deionized/distilled water and stain for 10 seconds.
3. Rinse the slides by immersing in deionized/distilled water for 30 seconds. You can also rinse or washing the slides with deionized or distilled water.
Thick blood films (only):
1. Allow the film to air dry thoroughly for several hours or overnight. Do not dry the film in the incubator or by heating, as this will fix the blood and interfere with the dissolution of RBC.
Note: If you need to diagnose malaria quickly, you can make a thick film thinner than usual, let it dry for 1 hour, and then stain it.
2. Immerse the thick film in distilled or deionized water for 10 minutes to change its color.
3. Allow the film to air dry thoroughly.
4. In a Coplin jar with anhydrous methanol, fix the air-dried film in anhydrous methanol for 30 seconds.
5. Allow the film to air dry.
6. Immerse air-dried blood film in the undiluted stain for 15 to 30 seconds (double the staining time for bone marrow smears).
7. Remove the color of the stained smears by immersion in distilled or deionized water and air dry
8. Let air dry in a vertical position.
Field stain: (thin film)
Materials
• Methanol (absolute)
• Field’s stain A und B
• Tube with water
• Staining dishes
• Filter paper
Procedure
A. By using methanol for 1 min you can fix a thin film.
B. Dry microscopic slide on filter paper
C. Dip slide in Field’s stain B (Eosin) for 5 seconds
D. Immediately wash with water
E. Dip slide in Field‘s stain A (Methylene blue) for 10 sec
F. Immediately wash with water
G. Dry thin films
Field stain: (thick film)
Materials
• Methanol No!
• Field‘s stain A und B
• Tube with water
• Filter paper
Procedure
A. Dip thick film in Field‘s stain A (Methylene blue) for 3 sec
Do not forget:
• Thick films need to be
• haemolysed and are
• therefore not fixed with
• methanol
B. Rinse immediately in tap water
C. Dip thick film in Field’s stain B (Eosin) for 3 seconds
D. Then rinse immediately with tap water
E. Let the slide carefully dry
Jenner stain:
• The Jenner stain Solution is a combination of various thiazine dyes in a methanol solvent
• Ionic and nonionic forces are involved in the binding of these dyes
• The dyeing solution has anionic and cationic properties
• The negatively charged phosphate group of DNA Bring purple polychromatic cationic dyes the nuclei
• The polychromatic cationic dyes staining the blue basophilic granules.
Immersion Staining Protocol:
• Thoroughly dry blood or bone marrow smears
• Absolute methanol should be used to Fix smears for 15 seconds to 5 minutes.
• Put smears in Jenners Stain Solution for 2 minutes for staining.
• Stain in a mixture of 50ml of Jenners Stain Solution, 75ml of PH 6.6 Phosphate Buffer Solution and 175ml deionized water for 5 minutes
• wash in standing deionized water for 1.5 minutes or rinse briefly in running deionized water
• Air dry smears
• Examine smears under a microscope.
Horizontal Staining Protocol:
• Put slide with thoroughly dried film in a horizontal staining rack
• Overflow smear with absolute methanol for 15-30 seconds and then drain
• Overflow smear with 1ml Jenners Stain Solution and let stand for 3 minutes
• Put 1mL of pH 6.6 Phosphate Buffer solution and 1 ml
deionized water to smear and wait for 45 seconds
• Rinse briefly with running deionized water
• Air dry and examine under a microscope
• Perform immunochemical staining procedure according to the manufacturer.
J.S.B. Stain:
Materials and reagents required:
• Eosin yellow (water-soluble)
• Methylene blue
• Potassium dichromate
• Di-sodium hydrogen phosphate (dihydrate)
• 1% sulphuric acid.
• round bottom flask (2 lit.)
• Healing mantle
• Distilled water
• Staining jars.
Staining technique:
• Make thin and thick smears from malaria cases on micro slides
• De-haemoglobinise the thick smear
• Fix the thin smear in methanol for one or two minutes
• Take 3 staining jars for J.S.B. I, J.S.B.II and tap water
• Immerse the smears in J.S.B. II for a few seconds and immediately wash in water
• Drain the slides free of excess water
• Immerse the smears in J.S.B.I for 30-40 seconds
• Wash well in water and dry
• Examine the smears under oil immersion.
Staining of Thick Smear:
• It may be stained with any of the Romanowsky stains
• listed above except that before staining, the smear is dehaemoglobinised by putting it in distilled water for 10 minutes.
Autostainers
• Currently, automatic staining machines are available which enables a large batch of slides to be stained with uniform quality.
Precautions in Staining of peripheral blood film
1) Dark blue blood film:
This may be due to excessive dyeing, insufficient washing or the buffer pH is inappropriate. In this red blood cells are blue, the nuclear chromatin is black, the neutrophil granules are over-stained, and the eosinophil granules are blue or gray.
2) Light pink blood film:
In this red blood cells are light red, nuclear chromatin is light blue, and eosinophils are dark red.
This may be due to insufficient staining, long-term cleaning, mounting of the film before drying, and inappropriate pH of the buffer.
3) Precipitate on the blood film:
This might be due to inadequate filtration of the stain, dust on the slide, drying during staining, and inadequate washing.








Comments
Post a Comment