Case study (45) - Burkitt’s lymphoma
Questions:
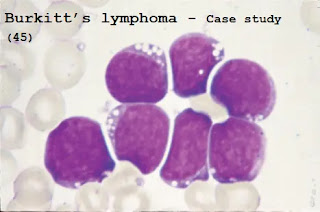
Q1. These blood films are from a 34-year-old woman who has a white blood cell (WBC) count of 63 × 109/L.
She was initially treated with four courses of intensive chemotherapy and entered complete remission.
She relapsed 6 months later and achieved remission again with chemotherapy, and then she received an allogeneic bone marrow transplant from all humans Leukocyte antigen (HLA) compatible equivalent.
What is the likely diagnosis at the presentation?
What chromosomal translocation is typically seen?
Q2. She recovered from the procedure but 9 weeks later she develops increasing shortness of breath. Her chest x-ray is shown below.
Q3. What is the differential diagnosis and how would you manage her?
Answers:
A1. The blood film shows large numbers of blast cells with intensely basophilic cytoplasm and vacuoles.
These features suggest B-cell acute lymphoblastic leukemia, but an identical appearance is seen in Burkitt’s lymphoma.
The cells are positive for the existence of monoclonal surface membrane immunoglobulin.
A2. Nearly all patients have a chromosomal translocation involving the c-myc oncogene (chromosome 8) and one of the loci for immunoglobulin genes (typically, the heavy chain locus on chromosome 14).
The event appears to be related to the abnormal and uncontrolled proliferation of primitive cells.
In this case, B lymphocytes can be seen.
Burkitt’s lymphoma usually occurs in African children who are chronically stimulated by B cells due to the malaria epidemic.
Most of these children also have recent evidence of B- lymphotropic virus, Epstein-
Barr virus. Although t (8;14) is the commonest translocation, variant forms include t (2;8) and t(8;22).
A3. She has developed bilateral diffuse pulmonary infiltrates in association with dyspnea.
Non-infectious causes, for example, pulmonary edema, chemotherapy toxicity and pulmonary hemorrhage are more common in the first post-transplant month.
However, at the 9th week, it is more likely to be the cause of infection (for example, virus-cytomegalovirus [CMV], herpes simplex, varicella-zoster, and respiratory syncytial virus), Pneumocystis carinii (despite the use of conventional Prevention of co-trimoxazole or pentatam has reduced this condition) and fungal or bacterial infections.
CMV seronegative bone marrow transplant recipients must receive CMV-negative blood components.
A doctor in the Intensive Treatment Unit (ITU) must perform blood gas/oxygen saturation analysis and clinical examination.
Accurate microbiological diagnosis should be sought, such as through possible transbronchial biopsy, sputum culture and cytology, blood, tissue, and urine culture, especially CMV for bronchoalveolar lavage (for example, by detecting early antigen fluorescent foci [DEAFF] Test or polymerase) chain reaction) and computed tomography (CT) scan to look for evidence of fungal or Pneumocystis infection.
Requires empirical broad-spectrum antibiotics and antiviral therapy (such as IV ganciclovir plus CMV hyperimmune globulin), as well as specific anti- pneumocystis (used with high-dose compound trimethoprim) and anti-fungal therapy (Intravenous liposomal amphotericin), should be considered.



Comments
Post a Comment