Thalassemias
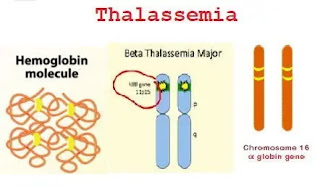
The hemoglobin molecule is composed of heme and globulin.
Globin Chains:
Are consists of four polypeptide chains: two alpha chains and two beta chains.
Alpha Globin: Coded for on Chromosome 16 (Each chromosome 16 carries 2 genes. Therefore, the total complement of a person' a genes is 4).
- Present in normal adult hemoglobin, A and A2.
Beta Globin: encoded on chromosome 11 in Hgb A
Delta Globin: Found in Hemoglobin A2- Presented in small amounts in all adults.
Gamma Globin: Found in Fetal Hemoglobin
Zeta Globin: Found in embryonic hemoglobin
Each alpha-globulin gene produces only half the protein of a single beta-globulin gene. This keeps the production of protein subunits equal.
Thalassemia occurs when the globulin gene fails, and the production of globulin protein subunits is unbalanced.
Thalassemias
Definition: It is a group of inherited autosomal recessive genetic defect results in a reduced rate of synthesis or no synthesis of one of the globin chains.
1-beta thalassemia - reduced beta chain synthesis.
2-alpha thalassemia - reduced alpha chain synthesis.
- This leads to the formation of abnormal hemoglobin molecules, which can cause anemia.
Epidemiology of thalassemia:
- α Thalassemia exists in the Mediterranean Basin (including Greece and Italy), West Africa, the Middle East (especially Saudi Arabia), China, and Southeast Asia.
- β thalassemia is most common in the Mediterranean Basin, especially Greece and Italy.
It is also found in parts of North and West Africa, the Middle East, India, China, and Southeast Asia, although α thalassemia is more common in these areas.
Alpha Thalassemia
Classification & Terminology of α Thalassemia
• Normal aa/aa
• Silent carrier - a/aa
• Minor -a/-a or --/aa
▪ α Thalassemia generally caused by gene deletions.
▪ α + chromosome produces some α globin (e.g. - α/- α).
▪ α 0 chromosome does not produce α globin (e.g. - -/ α α).
|
Condition
|
Genotype
|
Clinical Feature
|
Newborn
Hb pattern
|
Hb pattern
>First Year
|
|
Hydrops fetalis
|
--/--
|
Fetal or neonatal death
|
Hb Bart’s >80% HbH
|
--------
|
|
HbH disease
|
--/-a
|
Chronic hemolytic anemia with splenomegaly
|
Hb Bart’s 20-40%
|
Hb H 10-40%
|
|
Thalassemia minor
|
-a/-a
or
--/aa
|
Slight anemia,
Micro, hypo RBC
|
Hb Bart’s 2-10%
|
No increase in hemoglobin A2 or F – No hemoglobin H
|
|
Silent Carrier
|
- a/aa
|
No hematologic or clinical
abnormal.
|
Hb Bart’s 1%
|
No increase in hemoglobin A2 or F – No hemoglobin H
|
|
Normal
|
a a/aa
|
No hematologic or clinical
abnormal.
|
Hb Bart’s 0-trace
|
Normal
|
Laboratory findings of Alpha-thalassemia
- Alpha thalassemia minor is more difficult to diagnose because the levels of HbA2 and HbF are not increased.
Frequently it is a diagnosis made by exclusion.
- Iron profile (serum iron, TIBC, and ferritin-exclude iron deficiency anemia.
- Prenatal diagnosis can be confirmed by CVS (chorionic villus sampling) or amniocentesis.
Beta thalassemia
Beta thalassemia is a form of thalassemia due to a genetic defect in the Hbβ gene (autosomal recessive gene) on chromosome 11.
Types according to mutations:
(β): Normal.
(βo): Complete elimination of β -chain.
(β+): Allow some β chain formation.
Types of beta-thalassemia:
1- Homozygous or double heterozygous: (Thalassemia severe or Cooley's anemia):
(βo/βo)= No β globin = No HbA
β+/βo or β+/β+
● Increased levels of HbF & HbA2 with an absent or low level of HbA leads to severe symptoms.
2- β- Thalassemia minor (Thalassemia trait):
- Only one of β globin gene bears a mutation heterozygous (β+/β or βo/β).
- It is the carrier state of β - Thalassemia.
● Asymptomatic or mild microcytic hypochromic anemia (↓MCV and ↓MCHC values).
● Splenomegaly.
● ↓ fraction of HbA <97.5%.
● ↑ fraction of HbF & HbA2 > 3.5% leads to mild symptoms.
● It needs no treatment.
3- β- Thalassemia intermedia:
- Intermediate conditions between major and minor forms
- Genetic defects: homozygous patients (β+/β+ genes) or heterozygous (βo/β+).
- Affected people can live normally, but may occasionally need blood transfusions, (In case of illness or pregnancy, depending on the severity of anemia)
Explanation of gene defects in β thalassemia:
|
β thalassemia major
|
β thalassemia intermedia
|
β thalassemia trait
|
|
Homozygous
βo/βo
No β globin No HbA
|
Homozygous
β+/β+
or
Heterozygous
β+/βo
|
Heterozygous
(one gene
normal)
β+/β or βo/β
|
|
|
It may associate as
β thalassemia major
or
β thalassemia minor
|
|
|
Severe symptoms
Need blood transfusion
|
manage a normal life
Occasional transfusions
(in illness or pregnancy)
|
Symptomless or mild anemia
Not need treatment
|
Cellular pathogenesis of β-thalassemia major:
● ↓β chain globin so excess production of α chain then precipitates in RBCs (inclusion bodies) leads to ↑the destruction of RBCs precursors.
● Destruction of RBCs precursors leads to ineffective erythropoiesis (BM)& short life span of circulating RBCs then sequestration in the spleen
● Splenomegaly and hypersplenism
● ↑Hb catabolism so ↑bilirubin then jaundice & gall stones.
● ↓Hb (hypochromia), ↓mature RBCs & shortened RBCs survival cause anemia leads to ↑erythropoietin from the kidney then massive expansion of bone marrow finally bone deformity & fractures, and extramedullary hematopoiesis.
● Repeated blood transfusions and GIT absorption of iron lead to iron overload (due to ineffective red blood cell production).
Clinical Picture of Beta thalassemia major - (Cooley anemia = Target cell anemia)
The clinical onset occurs gradually
Clinical features
Starts after the age of 6 months
1-Severe anemia: easy fatigability, breathlessness, palpitation, and jaundice.
2- Excessive RBCs destruction and extramedullary hemopoiesis lead to an enlarged spleen and liver (hepatosplenomegaly.
3-Mongoloid features (thalassemic facies) due to Bone marrow expansion caused by strong bone marrow hyperplasia.
Thalassemic facies: Thinning of the bone cortex: In the maxilla, the face and skull can cause severe bone deformities, including enlargement of the frontal lobe and the protruding skull.
4- Strange skin color: (a combination of jaundice, pallor, and melanin deposition (white iron deposition can damage the skin and enhance the ability of melanocytes to produce melanin).
Laboratory diagnosis
1- Peripheral blood:
● Severe hypochromic, microcytic anemia (poikilocytosis and anisocytosis)
● Raised reticulocytes percentage
● Normoblasts
● target cells.
● Blood indices: MCV & MCH ↓ ↓ & decrease MCHC.
2- Hemoglobin electrophoresis: HbA is decreased or absent, HbF and HbA2 are increased (it may be normal, low, or increased).
- In thalassemia major:
Hb F: 98%
Hb A2: 2%
- In thalassemia minor:
Hb A: 10-20 %
Hb F: 70-80 %
Hb A2: variable
3-Hyperplastic Bone Marrow:
Due to ineffective erythropoiesis and RBC precursors destroyed.
Blood film of B-thalassemia
4- X-ray: skull shows hair on end appearance. Long bones showing thin cortex & wide medulla.
5- Iron studies (iron, transferrin, ferritin) can be used to rule out iron deficiency.
6. A definite diagnosis requires genetic testing (using Southern blot or polymerase chain reaction).
Iron studies
|
|
Iron Deficiency
|
Chronic Disease
|
Thalassemia
|
|
Hemoglobin
|
N or ↓
|
↓
|
N or ↓
|
|
Serum Fe
|
↓
|
↓
|
↑ or N
|
|
Transferrin Receptor
|
↑
|
↓ or N
|
N
|
|
Transferrin Saturation
|
↓
|
↓
|
N
|
|
Ferritin
|
↓
|
↑or N
|
↑ or N
|
|
MCV
|
↓
|
↓ or N
|
↓
|
|
Marrow Fe
|
↓
|
↑
|
↑
|
● In thalassemia minor, Hb and Hct decreased, but the RBC count is within the normal range, resulting in discordance in the indices.
(MCV is slightly lower, MCH is lower, but MCHC is close to normal). In addition, RDW is normal).
● On the contrary, in iron deficiency, the RBC count is usually relatively low, and the effect of anisocytosis increases significantly, so the indices remain the same, while the RDW increases.
● Use Mentzer's formula to distinguish thalassemia mild from iron deficiency, as shown below:
MCV If the result is <13 thalassemia minor.
RBC count.
Management of thalassemia major
1- Regular blood transfusion to keep hemoglobin above 10 g/dL.
2- Splenectomy.
3- Iron chelation therapy is necessary to prevent iron overload.
4- If you have a poor diet, you should take folic acid regularly.
Complications of Thalassemias
The complications of thalassemia are linked to four factors:
• Chronic anemia: leads to growth retardation, delayed sexual maturation, cardiac dilatation, and congestive heart failure, decreased work capacity.
• Marked expansion of the bone marrow: The bone marrow becomes greatly expanded due to obvious erythroid hyperplasia, the bone marrow becomes greatly expanded.
The diploic spaces on the skull become wider, showing the characteristic "hair on the head" appearance on radiographs.
The frontal bone Hypertrophy leads to frontal bossing.
The maxillae hypertrophy causes protruding cheeks and malocclusion of teeth, forming a typical "chipmunk" look.
Thinning of the cortex of the vertebrae and long bones can lead to fractures.
Extramedullary hematopoiesis leads to enlargement of the spleen and liver.
Extramedullary hematopoietic lesions may occur in soft tissues (myeloma tumors), and paravertebral masses may cause compression of the spinal cord.
Iron overload: There is chronic excess absorption of iron by the gastrointestinal tract, driven by chronic erythropoiesis, and this is exacerbated by RBC transfusions.
Iron deposits in the heart can cause cardiomyopathy and arrhythmia.
Deposits in the liver can cause portal fibrosis and may lead to cirrhosis.
Liver cirrhosis can lead to developing hepatocellular carcinoma.
• Chronic hemolysis: Chronic hemolysis leads to splenomegaly, hepatomegaly, and bilirubin gallstones.
Hypersplenism may develop, increasing transfusion requirements.





Comments
Post a Comment